DECIDE improves the efficiency of the AIMES Streamline application
The EU Horizon 2020 DECIDE consortium has begun using the Streamline application to deliver a rigorous use case validation of the DECIDE Multi Cloud selection, contracting, architecting, deployment, optimisation and monitoring suite of tools.
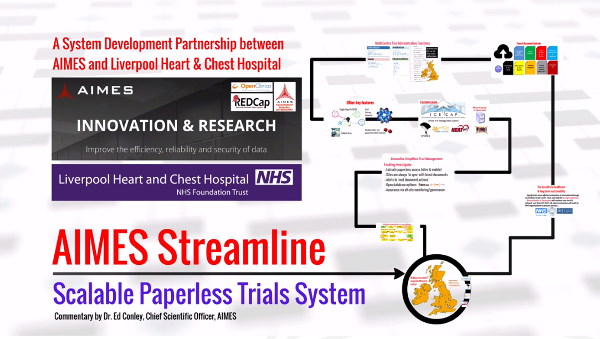
Streamline was co-created by AIMES, one of the DECIDE consortium partners, collaboratively with a specialist UK National Health Service (NHS) partner, the Liverpool Heart & Chest Hospital. The concept of using DECIDE to enhance the usability and productivity of Streamline has been expressly embedded in Streamline’s development philosophy. Streamline is a solution which establishes, sustains and archives clinical/medical (in this case NHS) cross-site evaluative research. Traditionally, approaches to multi-site studies have been costly and inefficient, unable to respond to increased demands from diverse innovation pipelines. Regulated research has detailed documentation requirements, which generates major storage security problems and bureaucratic delay for participating sites. Therefore, innovation is constrained by the individual centres capability to consume, analyse and archive large amounts of clinical information.
Streamline is a secure e-trial document management system, enabling distributed management of clinical-trial data. Current practice in large scale-scale, multi-centre clinical trials are often wasteful, accumulating a significant cost overhead. Trial protocols, staff records, training and governance information are typically maintained by participant sites in paper form, which is not efficient for the NHS. The governance and knowhow from the LHCH and AIMES partnership combined with technical expertise is used to create a trustworthy research environment, and it is currently deployed in 47 individuals, including cloud, environments. The benefits of employing Streamline in a research environment include: Reduced administration costs to keep protocols up to date, increased visibility of the research data for adaptive trial designs, easier data collection for medical professionals, making clinical trials paper-free and guaranteeing failsafe access to current documents and legacy versions, appropriate access from any web-enabled device, allowing remote-view and monitoring of research governance, for cost efficiency and assurance.
Quote from Ian Kemp (Research IT and Systems Lead), ICE CAP Research Group: “The online trial management system is a sea-change in trial governance. It relieves individual trial staff of the burden of site file management and provides many useful to the tools to the system administrators”.
From the inception of the DECIDE project, consortium partners were aware that the successful delivery of a robust, clinical/medical use case validation would provide an early opportunity to demonstrate the credibility of DECIDE in this, potentially mission critical environment. It was equally clear that Streamline’s requirement for deployment across a number of cloud environments, often from different Cloud Service Providers (CSPs), provided an effective validation of DECIDE’s underlying premise that efficiencies in the deployment and monitoring of systems across multiple clouds could be achieved without compromising efficiency or security. Accordingly, formal interaction between the two projects has taken place since the commencement of DECIDE and the Streamline system requirements were used to inform the development of the DECIDE tools.
Whilst Streamline is only one of the three use cases selected to deliver diverse, real world, validation of the DECIDE tool suite, a successful trial will make a positive contribution to the exploitation of DECIDE in the Health and Care sectors.
- root's blog
- Log in to post comments